-
PDF
- Split View
-
Views
-
Cite
Cite
A. M. Lovitz, A. M. Sloan, R. L. Rennaker, D. A. Wilson, Complex Mixture Discrimination and the Role of Contaminants, Chemical Senses, Volume 37, Issue 6, July 2012, Pages 533–540, https://doi.org/10.1093/chemse/bjs006
Close - Share Icon Share
Abstract
Rats were trained in a 2-alternative odor choice task to discriminate between a 10-component odor mixture and the same mixture with one component removed and replaced with 1 of 3 concentrations of a different monomolecular odor (contaminant). All stimuli were presented within a training session, thus the rat essentially had to learn to discriminate the 10-component mixture from “not” the 10-component mixture. Rats performed most poorly discriminating the complete mixture from the mixture with one component removed and no contaminant added. As the concentration of the contaminant increased from 10 ppm to a concentration equal to the other components (100 ppm), discrimination improved linearly. In analyses of individual differences, rats that spent more time in the sampling port (sampling and making a decision) were more accurate than rats that spent less time. Together, these results emphasize the balance between perceptual stability and perceptual discrimination expressed by the olfactory system dealing with dynamic mixtures and the robust effects of contamination on those processes. In addition, they provide further support that modification of sampling/decision time is a strategy used by rats to deal with difficult discriminations of complex odors.
Introduction
Odor mixtures are often perceived as unitary objects, and humans and other animals have difficulty identifying components within mixtures having more than 3 or 4 components (Staubli et al. 1987; Laing and Francis 1989; Jinks and Laing 2001). Although opportunities for interaction between individual odorants in a mixture exist in the olfactory epithelium and olfactory bulb, recent work has described a unique role for olfactory cortex in the synthesis of odor objects (Barnes et al. 2008; Chapuis and Wilson 2011; Chen et al. 2011). The piriform cortex serves as a pattern recognition device for spatiotemporal patterns of olfactory bulb activity and can store representations of familiar patterns (odor objects) in distributed ensembles of neurons (Haberly 2001; Wilson and Sullivan 2011). The memory component of this process allows for pattern completion in the face of slightly degraded inputs, with cortical ensembles ignoring minor deviations from a familiar pattern and responding as if the entire pattern was present. In rodents, piriform cortical ensemble activity predicts behavioral discrimination, thus odor mixtures that the cortex fails to distinguish due to pattern completion are difficult for the animal to behaviorally discriminate (Barnes et al. 2008; Chapuis and Wilson 2011).
An interesting phenomenon in piriform cortical and behavioral mixture discrimination is the effect of adding a novel contaminant to the mixture. For example, animals have difficulty discriminating a 10-component mixture from the same mixture with one component missing (90% overlap). However, if the missing component is replaced with a different novel component (contaminant; still 90% overlap), the animals and cortical ensembles easily distinguish this mixture from the standard mixture (Barnes et al. 2008; Chapuis and Wilson 2011). Contaminant effects on mixture perception and contaminant identification have been major areas of interest in studies of flavors and fragrances (Shipton et al. 1970; Ezquerro and Tena 2005; Honkatukia et al. 2005; Pickering et al. 2008; Weekes et al. 2010; Bonneau and Chevillon 2012). The addition of very small amounts of contaminants to wine or other food odors can have dramatic effects on their overall perceptual quality. These findings appear difficult to reconcile with the view of synthetic odor object perception but seem consistent with a system that can detect spoilage or contamination of foods.
Here, we further explored the ability of rats to detect contaminants in complex odor mixtures by replacing a single component of a 10-component mixture with a different molecule of varying concentration. In addition to looking at the ability to discriminate contaminated mixtures, we also quantified odor-sampling times (i.e., time in sampling port) to determine whether animals differentially sampled odors in the task related to their similarity. Work from several labs suggests that modulation of sampling time may be a behavioral strategy to maximize success on difficult discriminations (Abraham et al. 2004; Rinberg et al. 2006), though this is controversial and may be task dependent (Uchida and Mainen 2003; Uchida et al. 2006). Using a wide variety of molecularly distinct contaminants, the results suggest that contaminants are easier to detect in a mixture than the loss of a component, although this is dependent on the identity of the component. Furthermore, animals that performed better in the discrimination task remained in the sample port longer than animals that performed more poorly, suggesting an advantage for longer sampling times in making difficult discriminations.
Materials and methods
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Texas at Dallas and are in accord with Public Health Service guidelines.
Subjects
Male Long–Evans rats (n = 28) ranging in age from 7 to 24 months and weighing 300–600 g were trained on a 2-alternative forced choice discrimination task for water reward. Rats were water deprived during the week, they received water as a reward in the behavior task (∼5 mL/Day based on 100 rewards/day). They were given water ad lib Friday afternoon once training was complete for the day and again removed Sunday morning. Rat weights were monitored and maintained 85% of their initial body weight. Food was given ad lib.
Behavioral training
The behavioral training cage included 3 nose ports, one in each wall of the cage, instrumented with IR beams. The fourth wall is the door of the cage and is not instrumented. The middle odor-sampling port was used to initiate trials and deliver odors. A water reward was presented at the right reward port when the animal engaged it following a target stimulus. A water reward was presented at the left reward port when the animal engaged it following a distractor stimulus.
The odor stimuli consisted of a 10-component mixture (10c) as the target. Each component odorant (see below) was diluted to 100 parts per million (ppm). The distractors were the 10c mixture minus one of the components (10c − 1) and 3 different mixtures of the 10c − 1 plus a contaminant replacement at 3 different concentrations: low, medium, and high (100 ppm) (10c − 1 + L, 10c − 1 + M, 10c − 1 + H). The low concentration was set at either 1 or 0.5 ppm, and the medium concentration was either 10 or 5 ppm depending on the odorant.
The animals were trained using 4 stages. Animals were provided water in a reward port for 15 min each day for 5 consecutive days to associate water with the reward ports. They were then trained to engage the odor-sampling port and wait for the target odor, which occurred at a random delay centering on 500 ms after odor port entry (Stage 1). The required hold time increased from ∼1 up to 5 s; if the animal held, water was automatically delivered to the right reward port of the cage. Once the rats consistently held in the odor-sampling port for more than 5 s, they were moved up to the next training stage.
In stage 2 of their training, the rats were exposed to the target odor (10c) and the 10c − 1 + H distractor. Rats placed their nose in the odor-sampling port and were randomly exposed to the 10c or 10c − 1 + H odor, after a 2 s of exposure time to the odor, water was automatically delivered to the right reward port for the target odor or the left reward port for the distractor (10c − 1 + H) feeder. The minimum required hold time was reduced to 500 ms, which was maintained throughout subsequent stages. In this stage, water was delivered regardless of the animal's choice. The animal's responses were monitored, and once they had a d-prime (d′) of above 1.96, the rats were moved up to the next training phase.
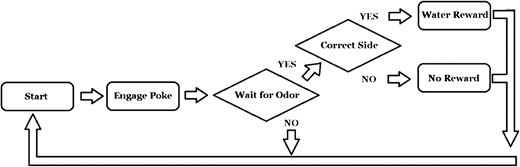
Stage 3 was exactly the same as stage 2, except water was delivered based on the animal making a correct decision (see Figure 1). That is, no water was delivered unless the animal's first choice was the correct reward port. Again, the criterion for graduating stage 3 was a d′ ≥ 1.96 (α = ∼0.05) for 2 consecutive days. After graduating stage 3, the rats moved to Stage 4.
Flow chart of the standard 2-alternative choice task. During stage 4 (testing), when data were collected, minimum required hold time was 500 ms.
In stage 4 (testing), the remaining 3 distractors were added to the stimulus set (10c − 1, 10c − 1 + L, and 10c − 1 + M). The animals were rewarded for going left on any of the distractors. All mixtures were randomly presented during a single training session, with approximately 50% of the trials 10c and the other 50% equally divided across the 4 distractors. Training sessions lasted 60–75 min during which the animals received at least 100 water rewards. The criterion for each session was a d′ ≥ 1.96. Animals were trained for a minimum of 5 days. The median number of rewarded trials per daily session was 136, and the median number of rewarded trials for a given odor set across all animals was 1112. Animals were given at least 2 days on free water before they started a new odor set.
Odor stimuli
A total of 7 different odor mixture sets were used, and most animals were trained on all 7 odor sets in random order. Animals completed testing on a given odorset, and all data included in the analyses here regardless of performance. Odors were delivered with a 10-channel olfactometer (Vulintus, LLC). Odorants were diluted in mineral oil to provide a concentration of 100 ppm for each component of the mixture based on vapor pressure. The standard 10-component mixture (10c) included the following monomolecular odorants (vapor pressure): isoamyl acetate (5.00 mm Hg), nonane (4.29), ethyl valerate (4.80), 5-methyl-2-hexanone (4.60), isopropylbenzene (4.58), 1-pentanol (6.11), 1,7-octadiene (6.15), 2-heptanone (3.86), heptanal (3.52), and 4-methyl-3-penten-2-one (6.69). This standard mixture was modified by removing, in different experiments, either ethyl valerate, heptanal, 4-methyl-3-penten-2-one, 2-heptanone, isoamyl acetate, isopropylbenzene, or 5-methyl-2-hexanone, to create a 9 component (10c − 1) odor mixture. The missing component was replaced with 1 of 5 contaminants: (+)-carvone (0, 1, 10, or 100 ppm; vapor pressure = 9.73), (−)-limonene (0, 0.5, 5, or 100 ppm; vapor pressure = 1.98), propyl butyrate (0, 1, 10, or 100 ppm; vapor pressure = 5.95), 3-methyl-2-buten-1-ol (0, 1, 10, or 100 ppm; vapor pressure = 6.90), or ethyl acetate (0, 1, 10, or 100 ppm; vapor pressure = 75.0). The standard mixture was the same as used in recent neurophysiological analyses of piriform cortical processing of odor mixtures (Barnes et al. 2008; Chapuis and Wilson 2011; Chen et al. 2011). The primary mixture included components that were all relatively similar in vapor pressure (3–7 mmHg) but that spanned a large range of molecular structure and human perceptual qualities (e.g., fruity, spicy, gasoline) and were all at a concentration of 100 ppm. The contaminants were chosen to also span a range of human perceptual qualities and be the same concentration or be 10% or 1–0.5% of the standard concentration.
Behavioral analyses
Correct and error responses were quantified for stage 4 sessions for comparison across distractors. In addition, total time spent in the odor-sampling port was quantified across odors and across correct and error trials. Given the large number of subjects, both mean and individual difference data were analyzed. Nonparametric methods were used for statistical analysis of subjects’ performance. Specifically, Wilcoxon matched-pairs signed-rank (MPSR) tests were used for pairwise comparisons and Friedman tests were used for analysis of variance. Bonferroni corrections were made to the Wilcoxon MPSR test for multiple comparisons on multivariate data sets to identify between-group effects. All error bars shown are the standard error of the mean.
Results
After sufficient training, all mixtures could be discriminated from the standard mixture. All animals were trained on more than one odor set. The task varied from our previous work (Barnes et al. 2008; Chapuis and Wilson 2011), in that in the present task, animals had to discriminate the 10c mixture from “not” the 10c mixture rather than make individual pairwise comparisons. The 10c − 1 and 10c − 1 + l, 10c − 1 + M were all presented for the first time on the first day of the testing stage (stage 4).
Mixture discrimination
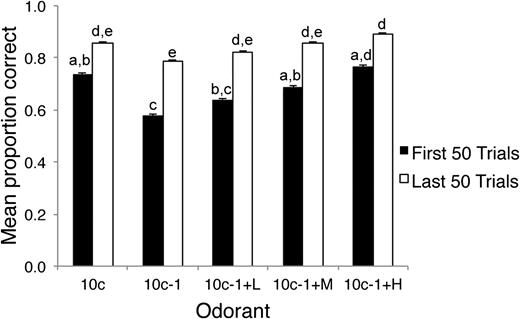
Figure 2 shows the results from the first 50 trials of the first day of testing (stage 4) and the last 50 trials of the final day of testing combined across all odor sets for 28 animals. The results show that animals make significantly more errors discriminating between the target and the 10c − 1 distractor on the first day of testing with an odor set than between the target and the contaminated mixes. Addition of a contaminant odor, even at moderate concentrations significantly enhanced discriminability from the target. Over the course of the first 50 trials, these effects were significant (Friedman test, χ2(4) = 23.01, P = 0.0001), and post hoc tests (Wilcoxon MPSR, Bonferroni correction) found that discrimination performance on 10c − 1 was significantly worse than the 10c, 10c − 1 + M, and 10c − 1 + H (αc = 0.0033). Performance on discrimination of the addition of the low (10c − 1 + L) concentration of the contaminant was intermediate. By the last 50 trials on an odor set (median 5 days of testing; Figure 2B), performance on all odorants was improved compared with the first 50 trials, improvements were significant except for 10c − 1 + H (Wilcoxon MPSR, Bonferroni correction). There was still a significant odorant effect in the last 50 trials (Friedman test, χ2(4) = 14.72, P = 0.005), but post hoc tests showed only one significant pairwise comparison between 10c − 1 and 10c − 1 + H (Wilcoxon MPSR, Bonferroni correction, αc = 0.0033).
Mean performance across all animals and odor sets for the first 50 and last 50 trials. During the first 50 trials with a new odor set, animals were significantly impaired in discriminating 10c from 10c − 1, whereas replacement of the missing component with a contaminant improved discrimination from 10c in a dose-dependent manner. Performance improved with additional training, with no differential performance between odorants during the last 50 trials. Bars labeled with the same letter are not significantly different from each other, whereas different letters signify significant differences. Error bars are standard error of the mean.
Differences in odors
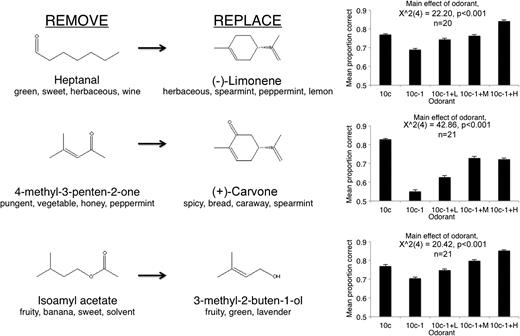
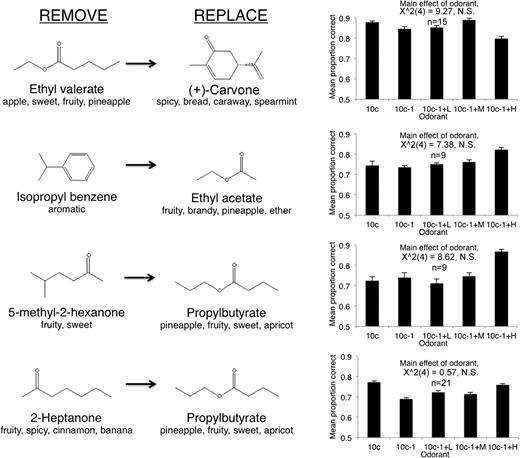
Although data from all odor sets were merged for the analyses in Figure 2, the pattern seen there was not consistent for all odor sets tested. Figures 3 and 4 show each version of the odor morphing and the associated first day behavioral performance. As shown in Figure 3, rats had significant difficulty detecting the removal of either heptanal, 4-methyl-3-penten-2-one, or isoamyl acetate (Friedman test, effect of odor for each odor set, P < 0.05). In contrast, as shown in Figure 4, rats did not have difficulty detecting the removal of ethyl valerate, isopropyl benzene, 5-methyl-2-hexanone, or 2-heptanone (Friedman test, effect of odor for each odorset, P > 0.05). The identity of the contaminants ((−)-limonene, (+)-carvone, propylbutyrate, 3-methyl-2-buten-1-ol, or propylbutyrate), however, did not differentially affect discrimination (Figures 3 and 4). The proportion correct did not significantly differ between 10c and 10c − 1 + H trials for any odor set (Wilcoxon MPSR, Bonferroni correction, not significant).
The identity of the removed component affected discriminability. Shown are 3 manipulations that resulted in impaired discrimination of 10c − 1 from 10c but reliable discrimination of the contaminated mixture from 10c. Compare with Figure 4. Molecular structures and verbal descriptors obtained from Glomerular Activity Response Archive (http://gara.bio.uci.edu/index.jsp). Error bars are standard error of the mean.
The identity of the removed component affected discriminability. In contrast to Figure 3, shown are 4 manipulations that resulted in reliable discrimination of 10c − 1 from 10c, as well as reliable discrimination of the contaminated mixture from 10c. Molecular structures and verbal descriptors obtained from Glomerular Activity Response Archive (http://gara.bio.uci.edu/index.jsp). Error bars are standard error of the mean.
Individual differences in performance
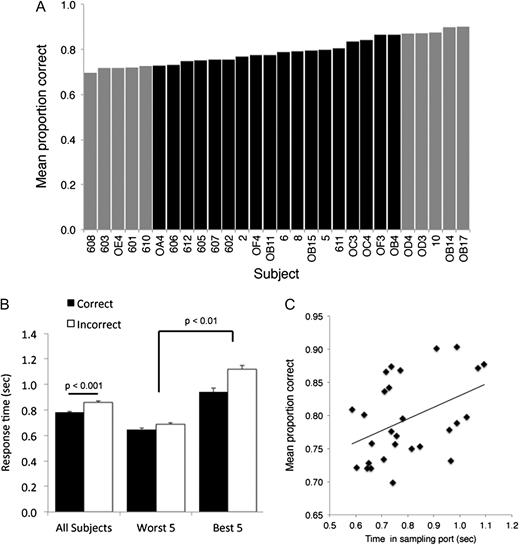
The large sample size allowed us to examine the effects of individual differences in performance (Figure 5A). In analysis of data merged across odor sets, there was a significant main effect due to rat (e.g., first 50 trials across merged odor sets, Friedman test, χ2(27) = 59.33, P < 0.001). As described below, these differences in performance correspond strongly with differences in sampling behavior.
(A) Individual animals varied in overall performance (proportion correct) across all trials and all odor sets. (B) Time spent in the sampling port varied as a function of overall performance and trial outcome. Animals spent significantly more time in the sampling port on trials that resulted in errors. However, animals that had overall higher correct performance (top 5 rats from A), sampled longer than animals with overall lower correct performance (bottom 5 rats from A). (C) Overall performance was significantly positively correlated with mean time spent in the sampling port. Displayed data are merged across all trials and all odorsets. Error bars are standard error of the mean.
Time in sampling port
The time an animal spends in the sampling port reflects a variety of factors including time spent actually sniffing the odor, past task requirements regarding holding time, current trial dynamics (e.g., stimulus onset, attention, etc.), and decision making. The minimum required hold time on testing days was 500 ms, however, in data merged across all trials and all odor sets, animals spent significantly longer in the sampling port on error trials than on correct trials (Figure 5B; Friedman test, effect of correct vs. incorrect, χ2(1) = 4.28, P = 0.039). This held true for both animals with overall high correct performance rates (top 5 rats from Figure 5A) and animals with overall poor correct performance (bottom 5 rats from Figure 5A; Friedman test, effect of best vs. worst rats, χ2(1) = 12.57, P < 0.001). However, top performing animals spent significantly longer in the sampling port than poor performing animals regardless of response outcome. In fact, performance was significantly positively correlated with sampling duration across animals (r = 0.42, P = 0.025; Figure 5C). These results suggest that animals that stay in the sampling port longer show better performance, and on difficult trials (i.e., those leading to errors), they spend even more time in the sampling port.
Discussion
The present results demonstrate that rats can reliably discriminate mixtures containing even small traces of contaminants from unadulterated complex mixtures. The addition of a contaminant to a complex mixture is generally easier to detect than the loss of a single component, consistent with a robust pattern completion process in olfaction. However, the identity of the missing component affected detectability, that is, not all components in mixtures of concentration-matched components were equal. Finally, analysis of individual differences suggests that animals that stayed in the sampling port longer performed better, and the more difficult the trial, the longer the animals chose to stay in the sampling port.
The present behavioral results are consistent with the view of olfactory cortex serving as a pattern recognition device capable of pattern completion and pattern separation functions (Haberly 2001; Barnes et al. 2008; Chapuis and Wilson 2011). Loss of a single component of a complex 10-component mixture was difficult to detect, suggesting this missing component was “filled-in” by the olfactory system to promote perceptual stability. Previous work has demonstrated that piriform cortical ensembles perform such pattern completion processes and only poorly distinguish between 10c and 10c − 1 (Barnes et al. 2008; Chapuis and Wilson 2011). However, loss of some components was more easily detected than others. Although components within the mixture were matched for concentration based on vapor pressure, they were not matched for perceptual intensity. Equal concentration odorants can vary in perceptual intensity due to differences in olfactory receptor sensitivity or differences in central processing. Thus, as previously reported (e.g., Staubli et al. 1987; Laska and Hudson 1993; Kay et al. 2005), some components may contribute more strongly to the overall perceptual quality of the odor mixture object than others. The mixtures used here are identical to those for which increasing sensory physiological data exist (Barnes et al. 2008; Chapuis and Wilson 2011; Chen et al. 2011), which allows mechanistic insights into perception and discrimination. However, future work will benefit from examination of complex mixtures whose components are matched or vary by, perceptual intensity, rather than chemical concentration. For example, the differences between odorsets observed here, with the loss of some components more easily detected than others, could be dependent upon differences in perceptual intensity of those components within the mixture.
Although loss of a single component was difficult to detect, replacement of that component with a single contaminant was reliably detected, in a concentration-dependent manner. As concentration of the replacement contaminant increased, discriminability from the standard mixture increased (Figure 2). This held true for a variety of molecularly distinct contaminants and did not appear to depend on which component was being replaced. These data are consistent with a shift in olfactory cortical pattern recognition toward pattern separation (and enhanced discrimination) as input patterns become more distinct (Barnes et al. 2008; Chapuis and Wilson 2011). These data are also consistent with human psychophysical data which often show robust effects of single contaminants on perception of food odors (Shipton et al. 1970; Ezquerro and Tena 2005; Honkatukia et al. 2005; Pickering et al. 2008; Weekes et al. 2010; Bonneau and Chevillon 2012). For example, the presence of even small amounts of androstenone or skatole strongly affect the odor quality of pork (Bonneau and Chevillon 2012), whereas the presence of compounds such as methylpyrazines can produce cork taints in wines (Chatonnet et al. 2010). It should be noted, however, that the human literature suggests both contaminant identity and concentration, as well as the mixture or food into which the contaminant is added, influence detectability (e.g., Pickering et al. 2008; Weekes et al. 2010). The present animal model, and the view of olfactory perception being driven by learned odor objects (Gottfried 2010; Wilson and Sullivan 2011), may allow a more mechanistic understanding of food odor taints.
Finally, based on individual differences our results suggest that animals that stayed in the sampling port longer performed better. As noted above, time spent in the sampling port reflects not only time spent actively sampling the odor but may also reflect a variety of factors including stimulus onset latency, task requirements regarding holding time, and decision making. Animals in our task spent considerable time in the sampling port (close to 1 s, whereas a minimum of only 500 ms was required) which is probably strongly influenced by earlier stages of training which required them to hold for up to 5 s before odor presentation. Nonetheless, across animals, holding time was significantly and positively correlated with accuracy. Thus, animals that spent more time in the sampling and decision process were more accurate. Interestingly, however, within animal analyses revealed that on the most difficult trials, that is, those that ultimately resulted in an error, the animals spent significantly more time in the sampling and decision process. Given that sniffing was not monitored here, these data do not directly address whether sampling longer is better (Uchida and Mainen 2003; Abraham et al. 2004; Rinberg et al. 2006). There is evidence that odor coding in both the olfactory bulb (Abraham et al. 2010; Cury and Uchida 2010; Shusterman et al. 2011) and piriform cortex (Rennaker et al. 2007) is rapid with significant decorrelation of odorants within the first sniff. However, the extended time spent in the sampling/decision phase noted here for more accurate rats, and the choice of all rats to spend even more time in those processes on the most difficult ultimately error-resulting trials suggests that time is an important factor in discrimination of complex overlapping odor mixtures.
Funding
This work was supported by funding from the National Institute on Deafness and Communication Disorders [R01-DC008982 to R.L.R. and D.A.W. and R01-DC003906 to D.A.W.].
The authors thank the anonymous reviewers for very helpful comments on the manuscript. Conflict of Interest Statement: Robert Rennaker is the owner of Vulintus LLC that manufactures the olfactometer and behavioral cages used in this study.